For MRI, there is a radio-frequency excitation pulse and then a period where signal is read. The duration between the excitation pulse and the center of the readout period is the echo time (TE). In many MRI studies, there is a single TE. This TE is set by the experimenter to to weight various factors of interest, which can be maximizing the contrast between gray and matter for structural imaging, or maximizing the Blood Oxygen Level Dependent (BOLD) contrast that is often used in fMRI. For BOLD fMRI, we typically set TE to be as close as possible to the T2* decay value for gray matter. For example a typical fMRI study on a 3T scanner, might acquire a single volume every 2 seconds with TE=28ms. A weakness of this approach is that other factors can affect the optimal TE for BOLD and undesired signals are also included for any given TR.
With multi-echo fMRI, for every excitation pulse, the same readout period is repeated as many times as possible before the next excitation pulse. That means, for each 2 seconds of acqusition, one could acquire three volumes with TE=(14ms, 28ms, and 42ms). By collecting multiple echoes, one can estimate the optimal TE for each voxel and use examine who signal changes across echoes to better isolate signal that is likely to be BOLD weighted.
1. Methods development
Prior to SFIMs work with multi-echo fMRI (Posse et al. 1999 and Poser et al. 2006) developed methods to take the weighed average of all echoes to benefit from a better estimate of T2* and improve data quality. This approach, sometimes called 'Optimal Combination,' produces a decrease in signal dropout and increase in the contrast-to-noise ratio (CNR). In SFIM, Prantik Kundu led work to develop a method that separates data into ICA components and the fits each component to models of T2* and S0 signals. Components that fit S0 signals, but not T2* signals were more likely to be artifacts, from the scanner or from head motion, which would be regressed from the data. This approach takes advantage of the physics underlying signal changes across echoes to identify noise. (Kundu et al. 2012 and Kundu et al. 2013).
The following image is from a review paper (Kundu et al. 2017) that shows example ICA components that fit well to a T2* (kappa) model (top row) and S0 (rho) model (bottom row). For this example, the lower row component would be regressed from the data as noise. 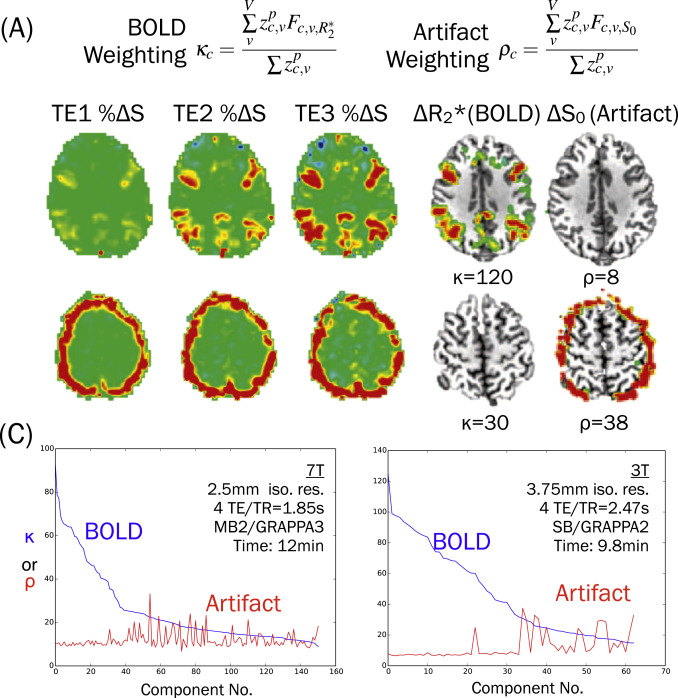
Continued work in SFIM has evaluated and expanded this ICA-based denoising approach. Evans et al. 2015 shows that multi-echo denoising has the potential to separate slow neural changes from other slow changes, like scanner signal drift. In single-echo fMRI, it is common to remove all slow drifts, which are assumed to be noise, which makes it difficult to study slow changes such as in studies of learning or studies that use pharmaceutical drugs. Gonzalez-Castillo et al. 2016 evaluates multi-echo denoise across multiple task designs to show that it is an effective tool for improving signal in small regions with high suseptability to cardiac pulsations, like the inferior colliculus. Cardiac gating us common for other MRI scans that benefit from synchronizing MRI acqusition with specific phases of the cardiac cycle, but it is rare with fMRI because variable spacing between volume aqusitions creates large artifacts. The above study shows that multi-echo denoising can remove these artifacts so that gated methods are possible with fMRI.
In addition to the ICA-based methods, we have collaborated with César Caballero-Gaudes' group to use multi-echo information to identify sparse peaks in fMRI series that are more likely to be neural in origin (Caballero-Gaudes et al. 2019 and Uruñuela et al. 2024). These methods can be used to identify timings of neural events, like cognitive state changes with no additional assumptions or measures of participant behavior.
Ongoing work includes efforts to combine multi-echo ICA denoising methods with other ICA denoising methods that fit data to signals such as head motion, cardiac or respiratory fluctuations, or CSF signals. We are evaluating these methods both for event-related task designs (Holness et al. 2022) and naturalistic movie viewing (Holness et al. 2023). We are also testing ways to alter ICA component estimation so that each component more purely contains BOLD or non-BOLD signals (Handwerker et al. 2018).
2. Software and Education
As we develop methods, we are also helping to distribute robust software and educational materials so these methods are widely available to the research community. This work centers on contributing to tedana which is both software and educational resources to help people better understand multi-echo fMRI and methods. Recent enhancements to the tedana software includes better recording of how the algorithm processes each dataset and a way for anyone to modify the ICA component selection process to fit specific needs or improve the overall method (tedana team 2023) and new ways to fit external time series to the components so that head motion and cardiac or respiratory fluctuations can be fully integrated into the component selection process (tedana team 2024).
Publications:
2024
Whole-brain multivariate hemodynamic deconvolution for multi-echo fMRI with stability selection
Medical Image Analysis
2021
The Journal of Open Source Software
2020
Network Neuroscience
2019
A deconvolution algorithm for multi-echo functional MRI: multi-echo sparse paradigm free mapping
NeuroImage
2017
NeuroImage
2016
NeuroImage
2015
NeuroImage
2015
Brain Imaging and Behavior
2015
NeuroImage
2013
Proceedings of National Academy of Sciences
2012
NeuroImage
Presentations:
June 2025
2025: Organization for Human Brain Mapping
June 2025
2025: Organization for Human Brain Mapping
June 2024
2024: Organization for Human Brain Mapping
July 2023
2023: Organization for Human Brain Mapping
July 2023
2023: Organization for Human Brain Mapping
June 2022
2022: Organization for Human Brain Mapping
June 2022
2022: Organization for Human Brain Mapping
Holness_OHBM_final.pdf
July 2020
2020: Organization for Human Brain Mapping
June 2018
2018: International Society for Magnetic Resonance in Medicine
June 2018
2018: Organization for Human Brain Mapping
June 2017
2017: Organization for Human Brain Mapping
June 2017
2017: Organization for Human Brain Mapping
October 2015
2015: Society for Neuroscience
October 2015
2015: Society for Neuroscience
sfn2015_poster_small.pdf
October 2015
2015: Society for Neuroscience
October 2015
2015: Society for Neuroscience
July 2015
Effects of multi-echo based denoising on reliability of a massively repeated block design task
2015: Organization for Human Brain Mapping
3931_gutierrez.pdf
July 2015
2015: Organization for Human Brain Mapping